KININX
Our offer consists of innovative patent-protected tests and expertise for drugs and medical devices for the pharmaceutical industry and for angioedema patients and clinical physicians. Angioedema expert: Our expertise is in the field of angioedema due to bradykinin. Mechanisms that interfere either with contact phase control, bradykinin production or degradation can lead to bradykinin accumulation on the endothelium and angioedema. The disease is characterized by sudden swelling of the hypodermis or submucosal tissues. It can be potentially life-threatening if it affected the upper airways. Therefore rapid and accurate diagnosis is essential. Angioedema can have sudden onset and can be inherited, acquired or provoked by certain types of medication such as those used for the treatment of allergies, diabetes or hypertension. Angioedema can be associated with either C1 Inhibitor deficiency or with a normal C1 Inhibitor function. KininX is the first company providing a complete solution for biological diagnosis of this disease particularly for angioedema with normal C1 Inhibitor function.
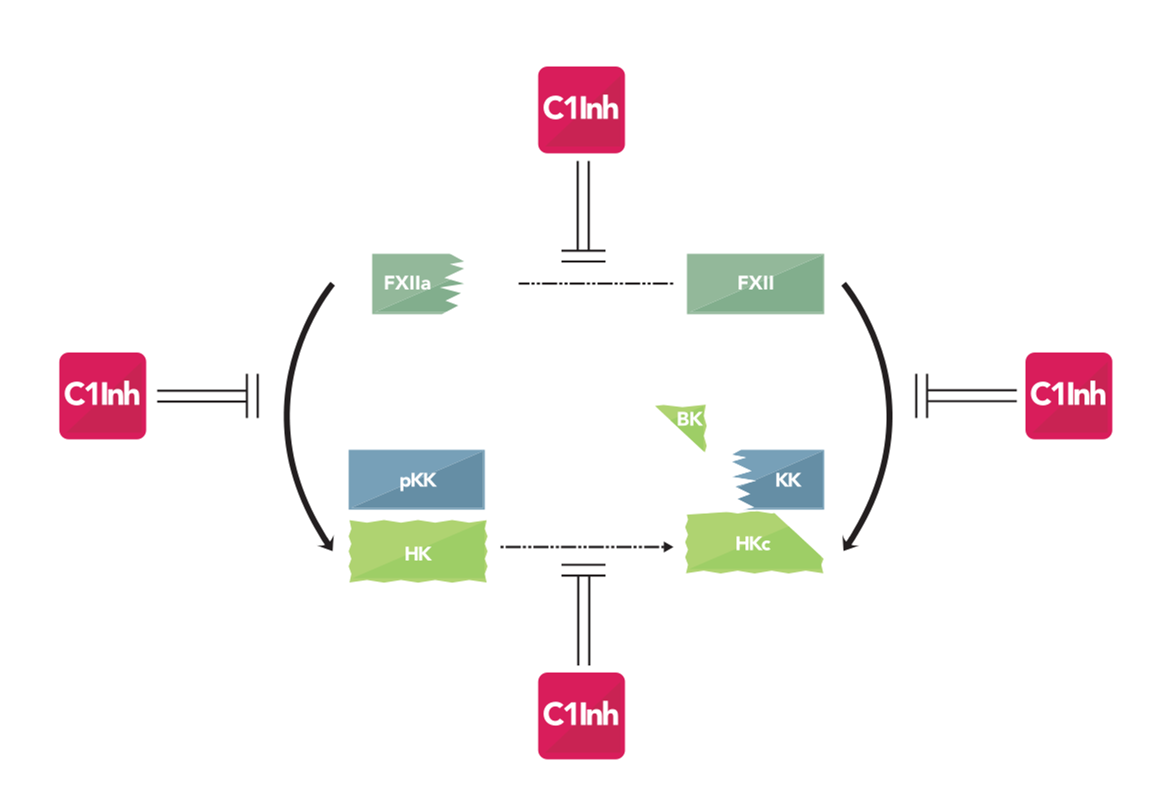
Thanks to our study of angioedema pathology we are experts in the kinin-kallikrein system. The mediators of this system, bradykinin, kallidin and their metabolites, are vasodilators and act on many different cell types. The kinin-kallikrein system is the topic of vast research due to its relationship to blood pressure and inflammation. Kinins are implicated in many different pathologies such as angioedema, Alzheimers disease, diabetes, obesity, hypertension, cancer etc. (White paper)
Pharma industry expertise:
We offer our expertise on the kinin-kallikrein system to pharmaceutical companies developing medicines for the treatment of angioedema, or medicines for which iatrogenic edema is a potential adverse effect or devices activating contact phase with subsequent anaphylactoid reaction hypotension and edema side effects. KininX offers many in vivo tools (animal models) and in vitro (contact phase reconstitution using purified components) to determine the impact of a therapeutic molecule on the kinin-kallikrein system and ex-vivo (deficient or normal plasma) solutions.
Expertise application:
- (1) KininX study medication for allergic desensitization which induced hypotensive shock. KininX demonstrated that the allergen, contained in the drug, activated contact phase protease responsible of hypotension and selected fractions with the less activation effects.
- (2) KininX study medications for angioedema treatment, helping companies to be more efficient during pre-clinical development about drug selection, formulation and doses and during clinical trial about definition of homogenate group of patients and demonstration of ex-vivo efficacity.
- (3) KininX study new anticoagulant drug and investigate its effect on kallikrein-kinin system and contact phase activation.
Angioedema diagnostic:
The existing biological diagnostic solutions for bradykinin angioedema are focus on complement (antigenemic C1 Inhibitor, C1 Inhibitor function with C1s as target and C4 antigenic level) while angioedema is related to kallikrein-kinin system activation. KininX developed new biological diagnosis solutions targeting kallikrein-kinin pathway (C1 Inhibitor function with kallikrein as target and kininogenase activity). Our diagnostic tests are the only way to understand angioedema pathology and to guide effectively the treatment of the disease. Illustrated by the study of Dessart et al., 2015, reporting that only 15 to 20% of angioedema are associated with C1 Inhibitor deficiency (inherited or acquired) whereas 59% are associated with disrupted kininogenase activity. KininX offer to patients and physicians medical biology kits in order to diagnose 77% of bradykinin angioedema.
KininX offers an innovative global solution for angioedema diagnostic by proposing two kits:
C1 Inhibitor function: Our kit for C1 Inhibitor measurement is based on an innovative method targeting kallikrein-kinin pathway activation and kallikrein activity. C1 Inhibitor is the major control of kallikrein-kinin system; its deficiency leads to excessive bradykinin production. Therefore testing for C1 Inhibitor function is an important test angioedema diagnosis. (Reference)


Kininogenases activity: The kit measures the capacity of plasma to produce bradykinin by measuring the capacity of enzymes to cleave kininogen. It is difficult to measure bradykinin because it is rapidly catabolized (T1/2 < 30 s), that’s why we developed innovative methods to measure bradykinin production. (Reference)


These assays are useful for the diagnosis of angioedema and the patient’s follow-up e.g. during treatment or pregnancy.
Our two kits offer a diagnostic solution for 77% of bradykinin angioedema patients. As for the rest of patients, KininX offers its expertise to help you in an efficient diagnosis.
Case Study:
- (1) J. a 10-year old boy presented in an emergency department with a subcutaneous angioedema affecting the face, an hand, a feet and the penis. He had an family history of angioedema (mother and maternal aunt). Intravenous H1 antihistamine and corticosteroid treatment did not improve symptoms whereas Icatibant was effective in 30 minutes. Concerning laboratory tests complement investigation were normal (C1Inh function targeting C1s, C4, C3 and CH50) while kininogenase activity was increased. No mutation on the F12 gene was funded. J was diagnosed with normal C1 Inhibitor hereditary angioedema, and icatibant was given at home in case of further attacks (Bergmann et al., 2013).
- (2) Mme T. is 26 years woman presented with recurrent face angioedema. H1 antihistamine was ineffective. She had no family history of angioedema. Any mutation on SERPING1 or F12 was identified. Hr laboratory tests for complement were normal (antigenic C1 Inhibitor and C4 and C1 Inhibitor function in regards to C1s). C1 Inhibitor function regarding kallikrein was decreased, 0.26 IU/mL (normal range for women is superior to 0,42 IU/mL) and spontaneous kininogenase activity was increased, 22 nmol/min/mL (normal range for women is inferior to 7 nmol/min/mL). She was diagnosed as normal C1 Inhibitor angioedema. She was improved using a treatement by tranexamic acid.
OUR TEAM

Takis Breyiannis
Chief Executive Officer & Founder

Arije GHANNAM
Chief Scientific Officer & Founder

Delphine Charignon
Product & Quality Manager

Christian Drouet
Scientific Advisor

Denise Ponard
Scientific Advisor
About Us
KininX was established in 2013 as a spinoff of the GREPI laboratory at the University of Grenoble and the Hospital of Grenoble. GREPI is a laboratory dedicated to the study of the mechanisms of inflammation. Kinins play an important role in inflammation and through our study of the kinin-kallikrein system we have become experts in the field of angioedema, an inflammatory disease that can be potentially fatal if not diagnosed and treated on time. We have developed a diagnostic for angioedema that allows for earlier detection and treatment of sufferers.
Our activity is based on 20 years of R&D and clinical experience. With our unique expertise we are able to offer 98% diagnosis of angioedema disease and guide treatment applications.
CONTACT
Publications
Diagnostic tests
- 1 Performance description of a new test for the C1 inhibitor function applicate to the angioedema diagnostic and follow up. Kallikrein use as the target is more relevant of contact phase activation implicated in the disease than C1s.
- 2 Performance description of a new test, the kinin forming activity, for the normal C1 Inhibitor function angioedema diagnostic.
- 3 Kininogenase activity application for angioedema with C1 Inhibitor deficiency.
- 4 Historical method for the C1 inhibitor function measurement, using C1s as target. This method shall be replaced by the new method using kallikrein target more pertinent of the system.
- 5 High molecular weight kininogen cleavage is analyse to highlight bradykinin production during an angioedema attack.
- 6 Relation between plasma kinin forming activity and VE-Cadherin release.
Bibliography review
- 7 Description of contact phase, its activation, its control and its development in the situation of hereditary angioedema.
- 8 Analyse of bradykinin antagonist application on the endothelium during the contact phase activation.
Genotype/ biological phenotype relationship in angioedema situation
- 9 First description of the c.983C>A F12 gene mutation causal for angioedema and its impact on contact phase.
- 10 and 11 Severity factor identification for hereditary angioedema with F12 gene mutation or C1 inhibitor deficiency.
- 12 Study of relationships between genotype and biological phenotype for angioedema.
Patient’s cohort suffering from angioedema analyse
- 13 Clinical and biological presentation of 50 women suffering from oestrogen-induced angioedema.
- 14 New classification of angioedema proposition to include angioedema with kinin catabolism deficiency. 2 The new in vitro test measuring kinin forming activity application on a large angioedema patient’s cohort suffering from hereditary angioedema with or without C1 Inhibitor deficiency.
Clinical cases of bradykinin angioedema
- 15, 16 and 17 Clinical cases where kinin metabolism investigation help to establish and define the angioedema diagnostic.
Help to the management and biological investigation of patient’s
